Shelf life, Storage and general considerations.
Shelf Life
Shelf life refers to the period
of time a sterilized or disinfected item is safe to use.
The time an item
remains safe for use is more event-related than time-related.
Actual sterility may be indefinite depending on the package materials, the item itself and the handling and storage. (Block)
Some items have a label claim of “Sterility guaranteed unless packaged is punctured or broken.” This labeling claims indefinite shelf like under the condition of proper handling and storage. There is no known reason to think items cannot be kept sterile indefinitely.
Some items may indicate a specific date after which sterility is no longer guaranteed. This expiration date may not be an indication of actual sterility but reflect other considerations by the manufacturer. Nor does this mean the items suddenly become unusable after that date. It generally means the package has gone through rigorous testing to simulate challenges equal to a specific period of time. The dates are to be respected and not ignored.
Maintaining product sterility is usually dependent upon your proper handling and storage to maintain the integrity of the package. Proper handling and storage is not optional and must be respected.
General considerations about proper handling.
Contamination can occur when microorganisms are introduced into a
package through puncture, dropping on the floor, rough handling,
bending, creasing, getting wet or damp, writing on the paper portion,
pressure and impacts.
Packages that are found to be creased, damp etc. are considered no longer fit for service and require re-processing. If pouches are roughly treated there may be no shelf-life at all.
Inspect all packages before use for integrity. Any packages that show signs of challenge must be marked for re-processing.
General considerations for storage.
Storage of all sterilized items should be designed to protect items from contamination. Containers and cabinets are recommended for items that are seldom used. Open shelves may be used but consideration must be given to traffic, ventilation around the items, especially air flow between the wall and the items and protection from housekeeping activities, such as having a barrier on the bottom shelf for protection during floor sweeping and away from areas that would likely get wet or moist, such as under sinks. These are general considerations that will be modified by the unique arrangement and layout of the storage area not had and fast rules.
Sterilized products should not be stored or kept in corrugated or
cardboard containers because those could potentially be contaminated and
generate dust.
The object is to limit dust and limit handling both intentional and
accidental.
Examples of poor handling and storage:
1) Repeatedly picking up many packages to thumb through them to find
the right size. This mishandling threatens the
integrity of the packages through friction of the packages being
rubbed together, hand pressure and air.
2) Stuffing or squashing them into a
tight container decreases package integrity.
Sterilized product must be retrievable with the least amount of handling
and the least amount of air and moisture exposure. This does not require
that all items be kept in closed containers or draws. Everything depends
on the likelihood of the packages becoming contaminated and maintaining
a sanitary environment.
Rotation of stock entails a system of adding newer sterilized items to an inventory to enable the use of the oldest first to prevent stale dating. When we go to the supermarket we always do the opposite: we look for and select the items that are freshest. In contrast, in dispensing we should select the oldest dated sterilized items first.
Packaged items
Package Dating, Expiration dating
All sterilized items not intended for immediate use should be dated.
In-house processing considerations
Since in-shop processing is not routinely biologically tested for every
run the date of sterilization should be put on the bag. The purpose of
dating is so that if the next test fails only those items sterilized
during that period have to be re-processed and not your entire
inventory. If you did not date the process date you would not know during
what period the items were sterilized and therefore your entire
inventory is suspect and
must be repackaged and reprocessed.
Many health departments
require biological testing monthly.
If your spore test were to fail, and you had not dated or lot numbered
your items, everything in your shop would need to be re-sterilized
before they would be considered safe to use.
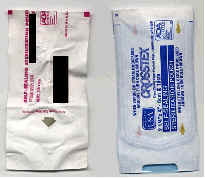
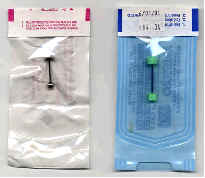
|
Could be better |
Good |
Could be better |
Good |
|
|
||
Transportation
Items being removed from storage should be transported so as to protect
the contents from inadvertent handling and environmental challenges
during the transportation.
Opening packages
When the item is to be used the package is inspected for intactness, not
wet or damaged, not torn or punctured and the Chemical Indicator (CI) on
the package shows process. Some items may have an Internal Integrator as
well. This should also be inspected.
The plastic side is peeled back and presented or the package is returned
to the field opened but the then remains on the package's sterile
internal side until needed. Change gloves and retrieve the item.
Needles are single use and immediately disposed of
after use into a sharps container as hazard waste.
Needles are all always single use and not to be re-processed.
Plastic Tubes are single use and disposed of after removal of the needle adn placed in normal trash. Plastic tubes are not hazard waste because you cannot squeeze blood or OPIM from them.
Re-processing
Stainless Steel tubes and grips and stainless steel tools such as
forceps
General safety in re-processing
Stainless steel items may be reprocessed following standard facility
recommendations for re-rprocessing and written procedures
for safety, such as, if splashing or splattering is expected (such as
when using a brush during cleaning) PPE including face protection, such
as a mask and goggles or a full face shield is required plus a full
fluid-resistant gown or covering that protects the skin and clothing
from becoming contaminated, hair covering, shoe covering, and heavy
rubber gloves, not latex examination gloves.
Grips and tubes are to be disassembled for cleaning. Rubber, silicone or
similar type grips that are stretched on should be removed and
sterilized unassembled because steam would not reach the contact
surfaces.
All stainless steel items are meant for autoclave or gas processing only, not dry heat.
Liquid Sterilization
Liquid sterilization products are not to be used.
The labels on sterilizing liquids clearly read that the use of those chemicals is a violation of federal law if they are used for any purposed other than what they are intended for. Sterilizing liquids are for use only on items that cannot be heat sterilized. All tattoo and piercing tools are heat sterilizable and excluded from liquid sterilization procedures by definition.
General comments
General considerations vs specific recommendations
The purpose of considerations is recognition that
rules should not be written to preclude technological development and
new techniques that become available.
For example, it would be an error to require any product by name be used
because a new product may become available that is better, or the
product recommended may actually have a previously undetected problem.
This actually happened in the mid west in which a state required the use
of TechniCare for skin disinfection. This product never had FDA approval
and the FDA forced the manufacture to stop selling the product. The rule
should have been written that a suitable skin disinfection product be
used and allow the facility to select the particular product that meets
the definition.